In the various processes of fabricating optical components such as lenses and prisms, surface deterioration is often encountered and recognized as dimming, staining and latent scratching. These surface defects are caused by chemical reactions of glass constituents with water in the surrounding environment or with detergents in the cleaning fluids.
Dimming
Polished glass exposed to high humidity and rapid temperature variations may "sweat.". Water vapor may condense to form droplets on the glass surface. Some of the glass components that dissolve in the droplets may in turn attack the glass surface and react with gaseous elements in the air (CO2, for example). Reaction products form as white spots or a cloudy film as the glass surface dries. This phenomenon is called "dimming.".
Fig. 2
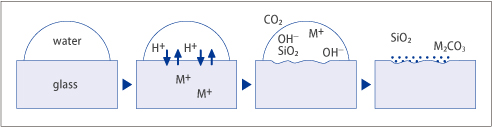
The resistance of a glass to dimming is expressed in terms of "water durability by the powdered method*(DW).".
Staining
When damage is caused to the polished optical glass surface by moisture and acid, the reflected light of an interference color may be seen on that surface.
This phenomenon is called "staining.". Water contact causes chemical reactions (ion exchange between cations in the glass and hydronium ions in water) that result in a silica-rich surface layer that causes an interference color on that layer.
Fig. 3
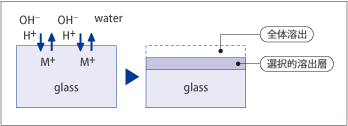
In this catalog, this resistance is expressed as "acid durability by the powdered method* (DA)" and "staining resistance by the surface method (TBlue)".
Latent Scratch
Fine scratches created on the glass surfaces during polishing will sometimes grow to a large size and become visible when surfaces are exposed to corrosive ions from inorganic builders found in cleaning detergents. This type of scratch is customarily called a "latent scratch.".
Fig. 4
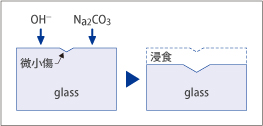
The inorganic builders such as Na2CO3, NaHCO3 or polymerized phosphate (mostly Na5P3O10) may attack glass in various ways: Through hydrolysis of the builders in the solution, the builders can form corrosive ions which attack the glass: hydroxyl ions, (OH-out of Na2CO3, NaHCO3), or polymerized phosphoric ions out of polymerized phosphate. Corrosion resistance to hydroxyl ions is expressed in terms of "latent scratch resistivity (DNaOH)," and is designated as "latent scratch resistance(DSTPP**) to polymerized phosphoric ions.
1. Dimming Resistance (Water Durability by the Powdered Method* (DW))
Glass is powdered and sieved to select particle sizes of 425 - 600μm of, powdered glass, weighed by specific gravity, which are placed in a platinum net basket and soaked in 80m pure water (pH=6.5 - 7.5) in a fused silica flask. The glass is then boiled for 60 minutes. The percentage of mass loss is measured and listed in this catalog, along with its class, and rated in Table 5.
Table5 Classes of Water Durability by the Powdered Method (DW)
| Class | Mass Loss(%) |
|---|---|
| 1 | < 0.05 |
| 2 | ≥ 0.05 - < 0.10 |
| 3 | ≥ 0.10 - < 0.25 |
| 4 | ≥ 0.25 - < 0.60 |
| 5 | ≥ 0.60 - < 1.10 |
| 6 | ≥ 1.10 |
2. Staining Resistance* (DA)
The acid durability rating employs a method of testing which is similar to water durability tested by the powdered method* DW, except that a 0.01mol/l nitric acid solution is used.
The percentage of mass loss is measured and listed in this catalog, along with its class, and rated in Table 6.
Table6 Classes of Acid Durability by the Powdered Method* (DA)
| Class | Mass Loss(%) |
|---|---|
| 1 | < 0.20 |
| 2 | ≥ 0.20 - < 0.35 |
| 3 | ≥ 0.35 - < 0.65 |
| 4 | ≥ 0.65 - < 1.20 |
| 5 | ≥ 1.20 - < 2.20 |
| 6 | ≥ 2.20 |
3. Staining Resistance by the Surface Method (TBlue)
A glass specimen with a 43.7mm diameter and approximately 5mm thickness, polished on both surfaces (with a total surface area of 30cm2) is immersed in pure water at 50°C, pH=7.0±0.2. The pure water is stirred well and circulated at a rate of 1l/min through layers of ion exchange resin. The specimen is then taken out of the water to examine the interference color in the stained surface under a 100W tungsten-filament lamp at predetermined intervals of time. The time required to form a bluish stained layer (n•d=120 - 130nm) is listed in this catalog, along with its class, in Table 7.
Notes: n denotes a refractive index and d denotes thickness of bluish stained layer.
Table7 Staining Resistivity by the Surface Method (TBlue)
| Class | TBlue |
|---|---|
| hours | |
| 1 | > 45 |
| 2 | 45 |
| 3 | 25 |
| 4 | 10 |
| 5 | 5 |
| † | Note |
Note :
Glasses in which the dissolution of the entire surface dominates and thus prevents observation of the bluish layers or glasses in which an irregular shift of the interference color is observed.
4. Latent Scratch Resistance (DNaOH)
A glass specimen with a 43.7mm diameter and approximately 5mm thickness, polished on both surfaces (with a total surface area of 30cm2) is immersed in a 0.01mol/l D NaOH solution at 50°C and then stirred well for 15 hrs. Mass loss per unit area is then measured and listed in this catalog, along with its class, in Table 8.
Table8 Latent Scratch Resistivity (DNaOH)
| Class | Mass Loss[mg / (cm2•15h)] |
|---|---|
| 1 | < 0.02 |
| 2 | ≥ 0.02 - < 0.10 |
| 3 | ≥ 0.10 - < 0.20 |
| 4 | ≥ 0.20 - < 0.30 |
| 5 | ≥ 0.30 |
5. Latent Scratch Resistance (DSTPP)
The latent scratch resistance, DSTPP, is measured in terms of mass loss per unit area. A glass specimen of 43.7mm diameter and approximately 5mm thickness, polished on both surfaces (with a total surface area of 30cm2), is immersed for 1 hour in a 0.01mol/l Na5P3O10(STPP) solution, at 50°C and stirred well. Mass loss per unit area is then measured and listed in this catalog, along with its class, in Table 9.
Table9 Latent Scratch Resistivity (DSTPP)
| Class | Mass Loss[mg / (cm2•h)] |
|---|---|
| 1 | < 0.02 |
| 2 | ≥ 0.02 - < 0.20 |
| 3 | ≥ 0.20 - < 0.40 |
| 4 | ≥ 0.40 - < 0.60 |
| 5 | ≥ 0.60 |
6. Intrinsic Chemical Durability to Water (D0)
An entire surface of glass, when immersed in water, may be susceptible both to leaching of soluble ions in the glass and simultaneously to disintegration of its network, (SiO2, B2O3), through hydrolysis.
The resistivity of glass to these reactions (leaching + disintegration) is directly related to the intrinsic chemical durability of the glass to water.
In this catalog, this resistance is expressed as "intrinsic chemical durability to water (D0).".
The intrinsic chemical durability to water, D0, is evaluated in terms of mass loss per unit of time per unit area[10-3mg/(cm2•h)]for a given period of time. Other test conditions are similar to those in TBlue Mass loss per unit, and then measured and listed in this catalog, along with their class, in Table 10.
Table10 Intrinsic Chemical Durability to Water (D0)
| Class | Mass Loss[10-3mg / (cm2•h)] |
|---|---|
| 1 | < 0.4 |
| 2 | ≥ 0.4 - < 5.0 |
| 3 | ≥ 5.0 - <10.0 |
| 4 | ≥10.0 - <15.0 |
| 5 | ≥15.0 |
7. Climate Resistance*(DH)
The classification in the next table is made by measuring the Haze (%) after the glass sample of 30x30x3mm with both sides polished is processed for 48 hours by temperature cycles of high temperature, high humidity provided for by the Japanese Optical Glass Industrial Standards JOGIS07-2009, in Table 11.
Table11 Climate Resistance class(DH)
| Class | Haze(%) |
|---|---|
| 0 | < 0.3 |
| 1 | ≥ 0.3 - < 3 |
| 2 | ≥ 3 - < 10 |
| 3 | ≥ 10 - < 30 |
| 4 | ≥ 30 |





